Bovine Abortion Caused by Leptospira spp.
Guy Sheppard, DVM, Gabriel Gomez, DVM, PhD, Pam Ferro, MS, PhD, and Megan Schroeder, PhD
Tissue from a cross-bred bovine fetus in the third trimester of gestation was submitted to the Texas A&M Veterinary Medical Diagnostic Laboratory (TVMDL) to investigate the cause of abortion.
According to the history provided by the submitting veterinarian, this animal was the second of two full term fetuses that had been born dead in the same herd. The necropsy findings in this animal were few and primarily consisted of a small amount of blood-tinged pleural fluid. There was evidence of scavenging as the abomasum was partially absent and no abomasal contents were available for culture. The spleen, lung, heart, liver, kidney, and small intestine were submitted fixed in formalin and sections of liver, kidney, abomasum, and spleen were submitted fresh to perform an abortion screen.
After microscopic evaluation of the fixed specimens, the major findings were intra-alveolar keratin squames and meconium pigment, interpreted to represent intra-uterine fetal stress, and moderate to marked cholestasis with prominent bile plugs in the bile canaliculi of the liver (Figure 1). Ancillary testing included bacterial culture of the abomasum, rtPCR for Neospora, Bovine Viral Diarrhea Virus (BVDV) and Bovine Herpesvirus-1 (BHV-1) in a sample comprised of liver and spleen, and rtPCR for Leptospira spp. in a sample comprised of liver and kidney. The bacterial culture yielded a mixed bacterial growth, likely due to contamination. Based on rtPCR testing, Leptospira nucleic acid was detected in the sample. PCR results for other pathogens were negative. The microscopic presence of cholestasis and the positive rtPCR test result for Leptospira are findings consistent with a diagnosis of leptospiral abortion.
Leptospirosis is a potentially zoonotic disease with clinical manifestations that depend on the animal species and the serovar (serological variant) of Leptospira interrogans. The most common L. Interrogansserovars involved in reproductive failure in cattle are Hardjo and Pomona; however, Icterohaemorrhagiae and Grippotyphosa can also affect the reproductive tract. In terms of the significance of this pathogen, Leptospira is one of the more commonly identified infectious organisms producing bovine abortion. Leptospires are shed in urine and infection occurs via penetration of intact mucous membranes or abraded skin. Leptospires prefer warm, wet climates, and they can survive in standing water for prolonged periods of time. Abortions generally occur in the second half of gestation and often occur several weeks after the organism invades the placenta and fetus. Abortion due to Leptospira is typically a manifestation of chronic infection and reproductive failure is frequently the only clinical sign observed in the herd. The abortion rate is often less than 10% with Hardjo infection, whereas infection with Pomona may approach 50%. Fetuses that are aborted due to Leptospira infection are typically autolyzed and icterus may be observed in some fetuses that are infected late in the gestation period. Although not present in all cases, the microscopic lesions that have been described in aborted fetuses are few and variable, but include renal tubular necrosis, interstitial nephritis, bile retention within canaliculi in the liver and non-suppurative meningitis.
Diagnosis of leptospirosis in cattle can be made by detecting a high level of antibodies to the organism in the serum from the cow or through antigen detection (e.g. PCR, rtPCR, etc.) testing of fetal tissues. The organism is shed in the urine of infected animals, thus personal protective equipment (PPE) should be utilized when working with cattle suspected to be infected in order to prevent human exposure. Additionally, minimizing exposure of susceptible animals to wildlife, rodents, and contaminated water sources, along with vaccination, should be employed to protect cattle from this disease.
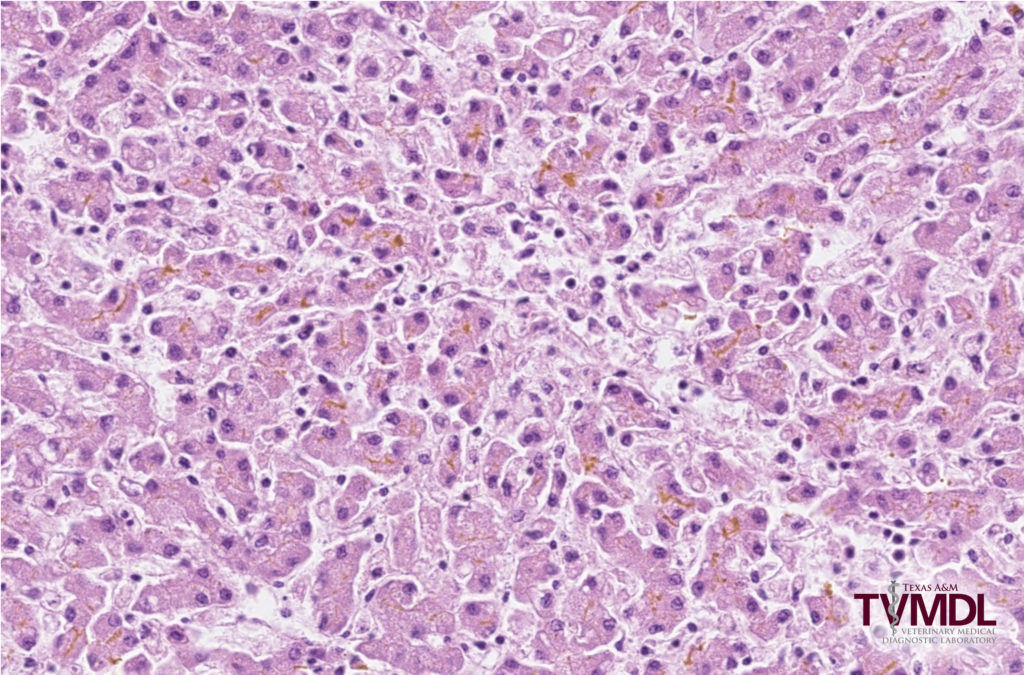
Figure 1. Photomicrograph of the liver depicting moderate to marked cholestasis with prominent bile plugs in the bile canaliculi.
References:
Barka, N. and Bernstein, M.D., Leptospirosis, Blackwell’s Five-Minute Veterinary Consult: Ruminant; Scott R.R. Haskell, DVM, editor. 2008 pgs. 471-473.
ML Anderson. Disorders of Cattle. Kirkbride’s Diagnosis of Abortion and Neonatal Loss in Animals. Bradley L. Njaa, editor. 2012. Ch. 2 pgs. 30-32.