Two porcine carcasses (carcass #1 and #2) and porcine tissue specimens from two additional pigs (tissues #3 and #4) were submitted to the Texas A&M Veterinary Medical Diagnostic Laboratory (TVMDL) for testing. All four animals were from different premises.
Porcine carcass #1 was 4-weeks-old and had a history of respiratory distress and being a “poor doer”. Additional information revealed that the sows on the premises were producing small litters and that the piglets were either stillborn, weak and/or exhibiting respiratory distress. On gross examination, the lungs were diffusely mottled tan and gray and failed to collapse. The parenchyma was firm and wet and had a consistency similar to that of the thymus. All the internal and external lymph nodes were moderately to markedly enlarged. No other pathologically significant findings were observed. Microscopic examination of tissues was not requested on this case.
Porcine carcass #2, age unknown, weighed 27 pounds and was from a barn experiencing a large number of deaths. Although no clinical signs were listed on the submission form, the submitting veterinarian mentioned clinical signs were consistent with Streptococcus infection. Grossly, the lungs failed to collapse and were diffusely meaty, wet, glistening and mottled tan to red. The internal and external lymph nodes were moderately enlarged. Microscopic evaluation revealed the interstitium of the lung was markedly expanded with infiltration of a moderate number of macrophages and lesser numbers of lymphocytes, plasma cells and neutrophils. These cells were accompanied with a small amount of fibrin and necrotic debris. The alveolar spaces contained necrotic debris, fibrin, mild acute hemorrhage, a moderate number of macrophages and scattered neutrophils. The lymphoid follicles in the spleen and tonsil were moderately to markedly hyperplastic. The interstitium of the heart had a small pocket of neutrophils and macrophages, consistent with a diagnosis of septicemia. Bacterial culture of the lung yielded Staphylococcus aureus and Escherichia coli.
Porcine tissues #3, age and weight unknown, had a reported history of repeated treatment for respiratory disease with no resolution of clinical signs. On gross examination, the submitting veterinarian described an “interstitial pneumonia” that affected approximately 60% of the lung fields. Sections of fresh and formalin fixed heart, liver, kidney, lung, lymph node, and spleen were submitted for testing. Microscopically, the interstitium of the lung was expanded with lymphocytes, plasma cells, macrophages and a few neutrophils. These inflammatory cells were accompanied by a small amount of necrotic debris. There was type II pneumocyte hyperplasia and the alveolar spaces contained mixed leukocytes and fibrin. The centrilobular areas in the liver exhibited hepatocellular necrosis attributed to hypoxia secondary to the lesion observed in the lung. Bacterial culture of the lung did not yield any bacterial growth.
Porcine tissues #4, age and weight unknown, reported a 4-day history of respiratory disease that appeared to have cleared after antibiotic treatment. However, soon after clearing the respiratory infection, the animal developed right-sided head tilt and ataxia (incoordination). The clinical condition of the animal improved with NSAIDS and antibiotic treatment but the following morning the animal was found down and paddling. The animal died shortly thereafter. Fresh samples from the lung and liver were the only tissues submitted for testing. Microscopically, the interstitium of the lung was expanded with moderate numbers of lymphocytes and macrophages and the alveolar spaces contained a small amount of necrotic debris, fibrin and a few neutrophils and macrophages. No significant lesions were observed in sections of liver. Bacterial culture of the lung yielded no significant pathogens.
Sections of the lung were submitted to the virology section for all four cases. These samples were tested via PCR for porcine circovirus type 2 (PCV-2) and porcine reproductive and respiratory syndrome virus (PRRSV), North American and European strains. In all cases, the tissues were positive for the North American strain of PRRSV and negative for the European strain of PRRSV and PCV-2.
Respiratory Disease in Swine
Respiratory disease is one of the most common disease syndromes affecting the swine population. Proper treatment and management rely upon a prompt and accurate diagnosis. Typically, arriving at a diagnosis starts with a history of clinical signs consistent with respiratory disease and/or identification of gross and microscopic lesions that suggest an infectious disease agent. At that point, suspicions of an infectious disease agent must be subsequently confirmed with additional testing at the diagnostic laboratory. Porcine Respiratory Disease Complex (PRDC) consists of both viral and bacterial components. The most common viral organisms are porcine reproductive and respiratory syndrome virus (PRRSV), swine influenza virus (SIV) and porcine circovirus type 2 (PCV2) while the most common bacterial organisms are Pasteurella multocida and Mycoplasma hyopneumoniae. Streptococcus suis, Actinobacillus pleuropneumoniae and Haemophilus parasuis.
A major pathogen in swine worldwide is PRRSV. First referred to as swine mystery disease (SMD) and subsequently a variety of other names including “blue ear disease” before the etiological agent was identified. PRRSV is an enveloped RNA virus in the family Arteriviridae and genus Arterivirus. This viral agent is an important cause of reproductive failure and interstitial pneumonia as well as a predisposing factor for bacterial pneumonia and septicemia. There are two predominant genotypes of this virus, type 1 (Europe) and type 2 (North America). The clinical manifestation PRRS is variable and is dependent on several factors including the age of the host affected, strain of PRRSV involved, level of immunity within the herd, and the presence of other pathogens within the herd. Viremia begins within 24 hours of infection and is able to persist in some animals for several weeks, thereby providing an ongoing source for susceptible hosts. This persistence combined with the virus’ ability to undergo significant variation (due to a RNA genome), creates a significant challenge in the treatment and control of the disease.
Transmission occurs by the transfer of body fluids (excretions and secretions) through direct contact, ingestion, inhalation, coitus, and bite wounds or trans-placentally during gestation. The target of initial infection and dissemination are the macrophages in nasal cavity, tonsil, or lung. From these sites the virus spreads to the regional lymphoid tissue which leads to infection of macrophages throughout the body. The direct cellular effect of infection is apoptosis of the infected and surrounding macrophages and lymphocytes, leading to immunosuppression and increased susceptibility to secondary infections. However, it is believed that the most significant mechanism of cellular injury associated with PRRSV infection is indirectly through the induction of inflammatory mediators, acute inflammation, and the degradative enzymes released by the inflammatory cells at the site of inflammation.

The primary targets of the virus are the reproductive and respiratory systems leading to clinical manifestations consistent with infection of these sites. These signs include anorexia and lethargy in all age groups. Reproductive failure characterized by late-term abortion, stillbirth, mummified fetuses or weak-born piglets may be observed in pregnant sows. In adult sows, PRRS infection in naïve herds may result in up to half of sows aborting with a mortality rate of up to 10%. In suckling pigs that are infected in-utero or during the neonatal period, fatal hyperpnea, dyspnea and lethargy may be seen secondary to interstitial pneumonia. Respiratory disease is also common in weaned and grower-finisher pigs.
PRRS can be divided into an acute and a persistent phase. During the acute phase, the virus is first phagocytosed by alveolar macrophages and then spreads to the bronchiolar-associated lymphoid tissue (BALT), alveolar septal macrophages, alveolar pneumocytes, and epithelial cells of the bronchi. The persistent stage of the infection is an important feature of PRRSV. In this stage, the virus establishes infections in the tonsil, spleen, lymph nodes, lung and in cells of the monocyte-macrophage system in other sites including the reproductive tract. Animals remain infectious for in-contact naïve pigs for a prolonged period after infection. There are reports that the virus remains in serum for 3 weeks; in nasal mucosa, pulmonary macrophages, and spleen for 4 weeks; and in oropharyngeal mucosa for up to 22 weeks after infection. Therefore, persistent infections are of epidemiologic importance in the maintenance of PRRSV infection in endemically infected herds. In addition, infection with PRRSV impairs host defenses, and increases susceptibility to infection with S. suis, H. parasuis, Salmonella enterica serovar Choleraesuis, and other opportunistic pathogens.
Notably, the clinical signs and lesions of PRRS are exacerbated in the event of subclinical or clinical infection with M. hyopneumoniae. This relationship is presumed to begin with Mycoplasma-induced proliferation and activation of macrophages. These macrophages are potentially used to enhance the replication or persistence of PRRSV. In contrast, infection with PRRSV does not appear to have a significant effect on the severity of Mycoplasmal lesions. Therefore, control of M. hyopneumoniae is one strategy for minimizing the effect of PRRSV in endemically infected herds.

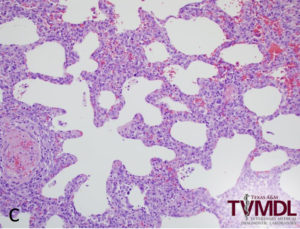
Gross lung lesions vary from undetectable to affecting the entire lung. The severity of the disease tends to be inversely proportional to the age of the animal. One of the most common findings is that the lungs fail to collapse and sometimes retain impressions of the ribs. The distribution of the lesion may be generalized, patchy, lobular, or diffuse. The areas that are affected are red or tan, have a firm texture and, on cut section, the interlobular spaces are expanded with edema. In pigs that develop bacterial bronchopneumonia secondary to PRRS, lesions of cranio-ventral consolidation may be superimposed on the diffuse interstitial pneumonia. The lymph nodes throughout the body, most notably the bronchial, mediastinal, cervical, and inguinal nodes, are enlarged, white to tan, firm, edematous and bulge on cut surface. Periocular and subcutaneous edema, pulmonary infarcts, myocardial necrosis, renal petechiae, and serous effusions into body activities are variable findings.
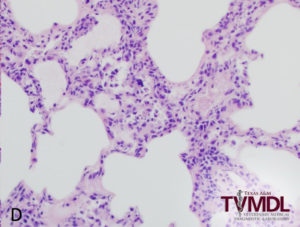
Microscopically, the alveolar septa in the lung are infiltrated with macrophages and lymphocytes and the alveolar spaces may contain a combination of macrophages, lymphocytes, neutrophils and necrotic cells with pyknotic nuclei or free chromatin. The presence of necrotic alveolar macrophages on sections of lung tissue is highly suggestive of PRRS. Unlike influenza virus, the bronchiolar epithelium is not affected by PRRSV. The lymph nodes, tonsils and spleen may reveal follicular and para-cortical hyperplasia, and apoptosis of follicular lymphocytes. Multinucleated cells, similar to PCV-2, may be present in cases of PRRSV infection.
In summary, PRRSV is a virus that remains a significant pathogen in swine and a challenge for management. Due to the complexity of the disease and multifaceted causes, diagnostics are important for determining the pathogens and affects. Diagnostic tests available at the Texas A&M Veterinary Medical Diagnostic Laboratory to aid in the diagnosis of PRRS include Necropsy, Histopathology, PCR, virus isolation, and Serology. The recommended samples for PCR testing include oral fluid, semen, serum, EDTA whole unclotted blood, lung, or fetal tissue. For serology, an ELISA and IFA test are available and serum is the required sample.
For more information about TVMDL’s test offerings, visit tvmdl.tamu.edu or call 1.888.646.5623.
REFERENCES
- askjpc.org
- Maxim MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th St. Louis, MO: Elsevier; 2016.
- Zachary JD, ed., Pathologic Basis of Veterinary Disease. 6th St Louis, MO: Elsevier; 2017.