Diagnostic Guidance: Postmortem Atlanto-occipital Cerebral Spinal Fluid Sampling in Large Animals
Jessie Monday, DVM, MS and Will Sims, DVM, MS
Any animal demonstrating neurological signs localized to the brain or spinal cord before death may benefit from testing of cerebral spinal fluid (CSF) collected at necropsy. If rabies is a differential, careful CSF sampling prior to removal of the head and/or brain allows for the potential of further diagnostic investigation if the brain tests negative for rabies. Additional testing of the fluid may include culture, cytology, serology, or molecular diagnostics depending on the clinical presentation and pre-testing differential list.
The following procedure is applicable to all animal species. The ease and amount of fluid obtained is proportional to the size of the animal with bovine/equine the easiest, and felines and small toy breed canines being the most difficult. For cytologic evaluation of CSF, fluid should be submitted in an EDTA tube and evaluated as soon as possible. CSF cellular degradation is rapid due to the low protein concentration.
Supplies:
- Necropsy knife
- Red Top Tube (RTT)
- Purple Top Tube (PTT)
- Sterile 6cc syringe with an 18 gauge by 1.5 inch needle
Procedure:
NOTE: CSF must be collected prior to disarticulating the head from the cervical vertebrae to ensure a sterile sample with minimal blood contamination.
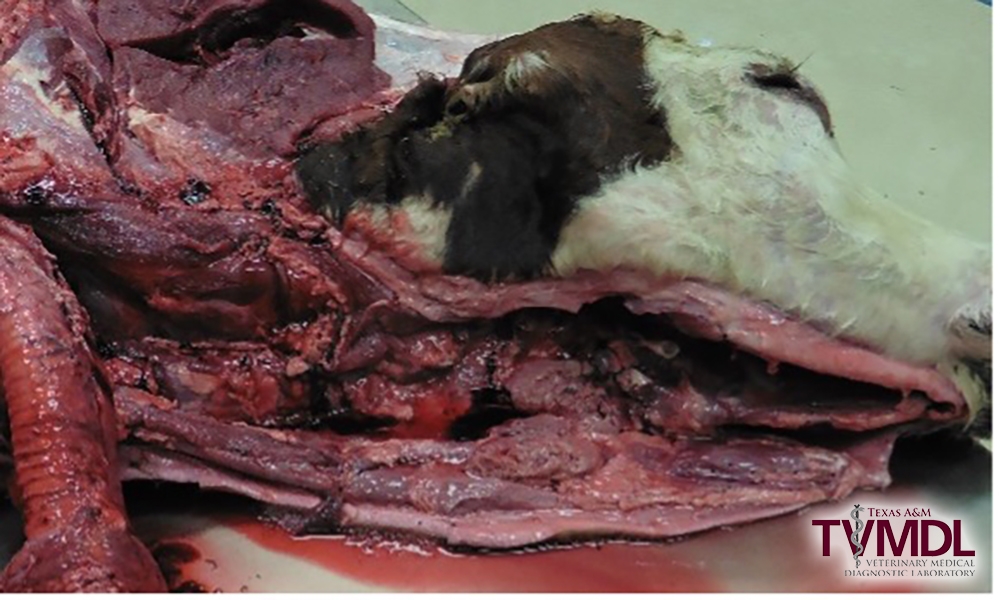
1. As part of the routine necropsy procedure once the cervical aspect of the pluck (tongue, larynx, and trachea) is removed, stop and collect the cerebral spinal fluid sample(s).
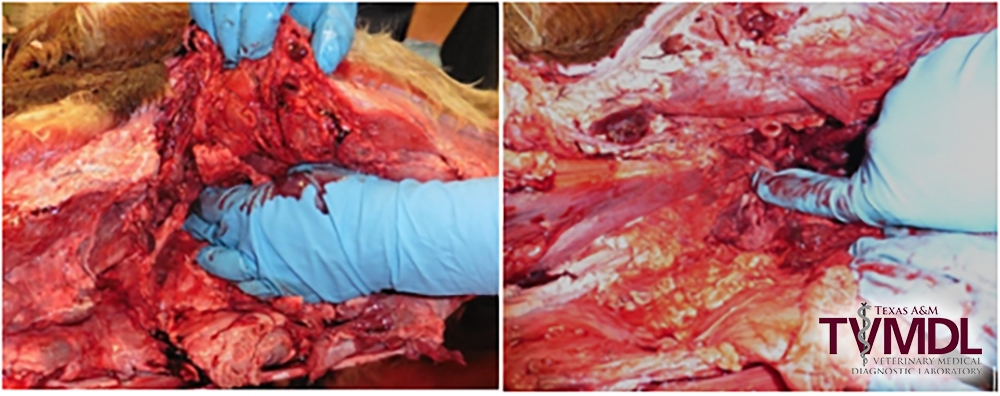
2. Locate the ventral aspect of the atlanto-occiptal (AO) joint by palpating for an opening on ventral midline through the muscles covering cranioventral aspect of the atlas as it articulates to the condyles of the occipital bone of the skull. Flexing the head ventrally while palpating will aid in identifying the AO joint location.
-
-
- If there is excessive muscle tissue, dissect some of the muscle layers to expose the joint capsule taking care not to enter the joint space and contaminate the CSF.
-
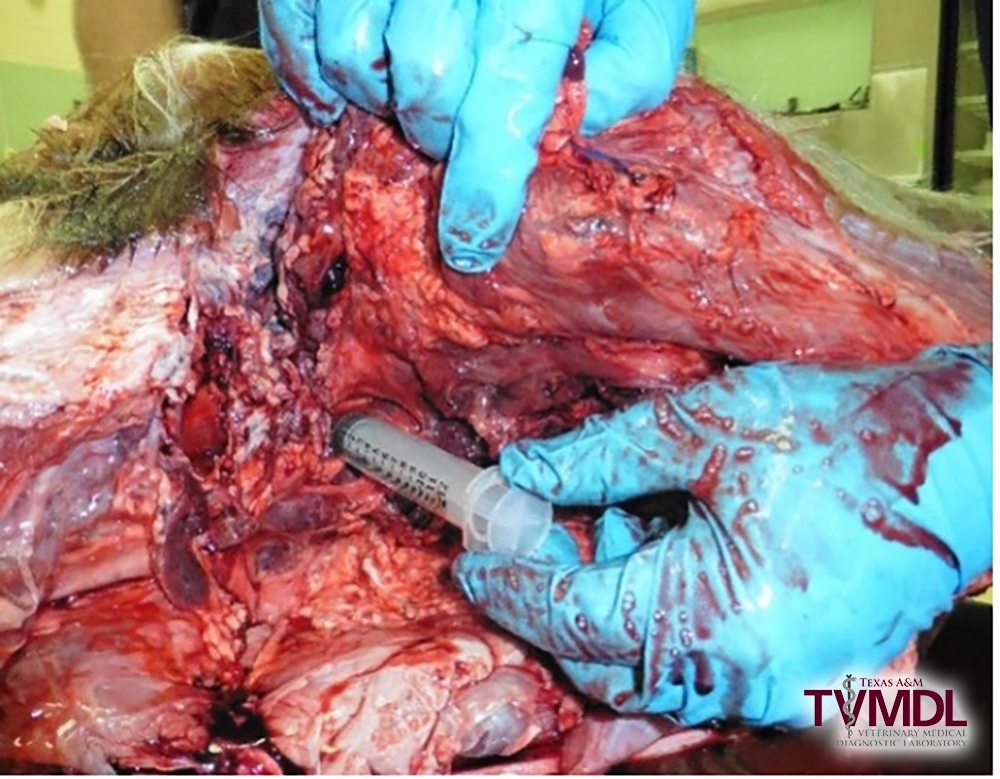
3. Holding the needle and syringe perpendicular to the spinal column, insert the needle into the space previously palpated.
-
- If you hit bone, redirect the needle until it falls into the space. The depth to insert the needle varies depending on the size of the animal, but on average an inch is typically sufficient to hit the AO joint space. It is important not to aspirate as you insert the needle to minimize blood and joint fluid contamination.
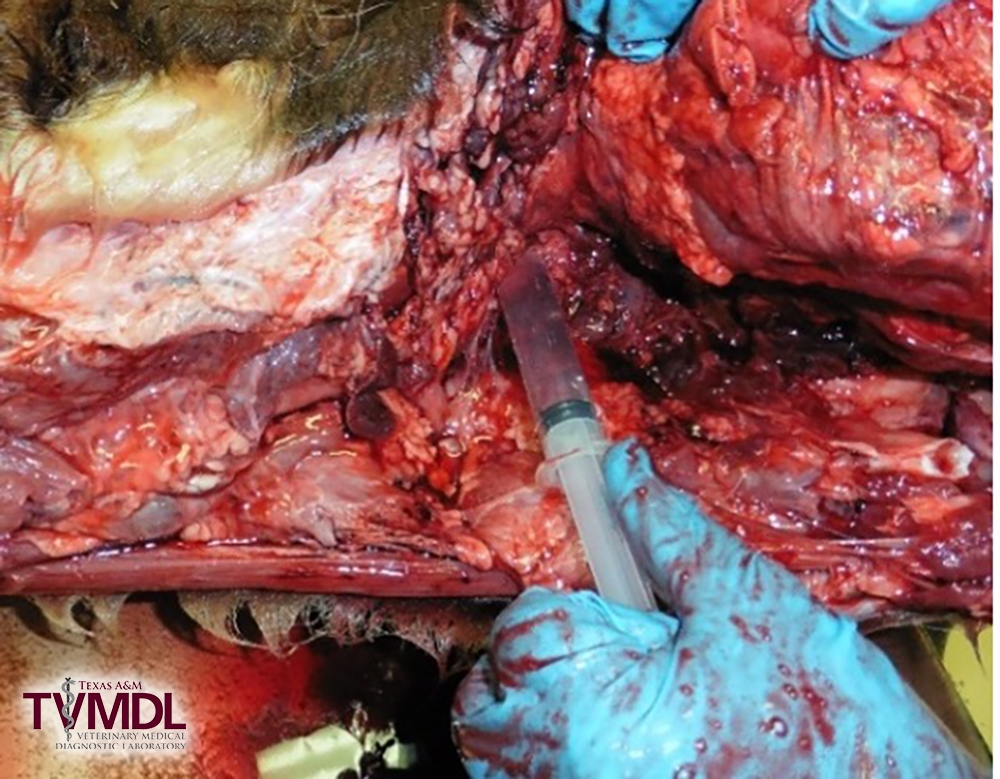
4. Once through the atlanto-occipital joint space into the cerebral spinal space slowly aspirate the fluid.
-
- If you get negative pressure and no CSF, continue to advance the needle until CSF fluid flows.
- Normal CSF fluid should be clear to champagne colored.
5. With adult cattle, there is typically enough fluid to fill multiple RTT and PTT tubes. With smaller calves, there may be a limited volume of fluid available for collection. Consider what diagnostics are needed and the amount of sample that is needed to perform each test. Distribute the available sample accordingly.
Additional information on tests that are run on CSF samples can be found using the following links:
- EDTA (lavender/purple top) sample collection tube:
- Additive free (red top) sample collection tube:
- Microprotein, Electrolyte Profile, Bacterial Identification – Companion Animal (Aerobic & Anaerobic Culture) or Bacterial Identification – Livestock (Aerobic & Anaerobic Culture), Listeria monocytogenes (PCR) [Referral], West Nile Virus (rtPCR), Equine West Nile Virus IgM (ELISA), Eastern Equine Encephalitis Virus IgM (ELISA), Equine Encephalitis Panel (ELISA), Western Equine Encephalitis Virus (VN), Equine Sarcocystis neurona (ELISA) [Referral], Cryptococcus neoformans Antigen (Latex Agglutination)